Pertussis Information for Health Professionals
- Pertussis Information for Health Professionals Home
- Managing Pertussis: Think, Test, Treat & Stop Transmission
- Pertussis Clinical Information
- Pertussis - Laboratory Testing
- Pertussis Treatment and Prophylaxis
- Follow-up Recommendations for Pertussis Exposures in the Health Care Setting
- Reporting Pertussis
Contact Info
Pertussis Laboratory Testing
Download PDF version formatted for print: Pertussis Laboratory Testing (PDF)
On this page:
Optimal timing for diagnostic testing (in weeks)
Polymerase Chain Reaction (PCR)
Serology
Culture
Tests not recommended for confirming pertussis
Submitting specimens and isolates to the MDH Public Health Laboratory (MDH-PHL)
Optimal timing for diagnostic testing (in weeks)
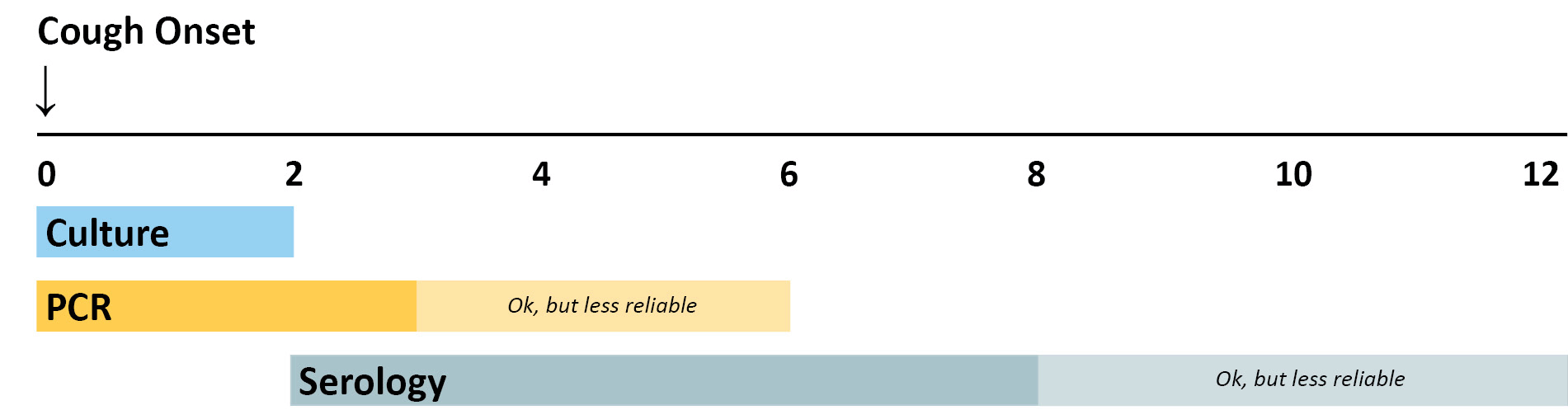
The breakdown of optimal timing is explained below.
Culture
Culture has excellent specificity, so it is very useful for confirming pertussis diagnosis when an outbreak is suspected. Many other respiratory pathogens have similar clinical symptoms to pertussis and co-infections do occur. Furthermore, obtaining isolates from culture allows for strain identification and antimicrobial resistance testing. Identifying which strains of B. pertussis are causing disease is of public health importance. Culture is best done from NP specimens collected during the first 2 weeks of cough when viable bacteria are still present in the nasopharynx. After the first 2 weeks, sensitivity is decreased and the risk of false negatives increases.
A negative culture result does not rule out pertussis infection. B. pertussis is most frequently recovered in the catarrhal or early paroxysmal stage of illness.
Specimen collection: Perform a nasopharyngeal swab in both nares. Gently insert the swab through the nostril to the posterior nasopharynx. Leave swab in place for 15-30 seconds, rotate, and remove.
Swab should be placed into appropriate transport medium or onto agar immediately. Choose transport medium and shipping conditions based on the length of time specimen will be in transit, contact reference laboratory for specific swab and transport medium requirements. Cotton swabs are not recommended for culture because cotton is harmful to B. pertussis. Regan-Lowe transport medium, or fresh Bordet-Gengou agar, casamino acid solution, and Amies transport medium with charcoal are all common media for the recovery of B. pertussis. Cultures are typically incubated 10-14 days, although results are generally available in 7-10 days.
Additional testing at MDH: All positive pertussis isolates are to be sent to the MDH-PHL for molecular sub-typing using pulse field gel electrophoresis (PFGE) and drug susceptibility testing, according to the Disease Reporting Rules (Minnesota Rules chapter 4605) on isolate submission.
- Molecular sub-typing: PFGE sub-typing is used to characterize B. pertussis strains present in Minnesota and to determine if certain strains are more likely to be associated with severe disease or vaccine failure. Sub-typing is also used as an adjunct to epidemiology to assess possible transmission patterns.
- Drug susceptibility testing: Drug susceptibility testing is performed to determine whether erythromycin-resistant strains are present.
Polymerase Chain Reaction (PCR)
PCR is a rapid test and has excellent sensitivity. PCR tests vary in specificity, so obtaining culture confirmation of pertussis for at least one suspicious case is recommended any time there is suspicion of a pertussis outbreak. Results should be interpreted along with the clinical symptoms and epidemiological information. PCR should be tested from nasopharyngeal (NP) specimens taken at 0-3 weeks following cough onset but may provide accurate results for up to 4 weeks. After the fourth week of cough, the amount of bacterial DNA rapidly diminishes, which increases the risk of obtaining falsely negative results. PCR assay protocols that include multiple target sequences allow for speciation among Bordetella species.
Specimen collection: Contact reference laboratory for specific swab and transport requirements. Calcium alginate swabs should not be used as they inhibit PCR.
Serology
A single-point IgG serology assay is available. This assay may be more useful than PCR in individuals presenting with a cough of greater than 2 weeks, especially for individuals not recently vaccinated against pertussis. The optimal timing for specimen collection is 2-8 weeks following cough onset—when the antibody titers are at their highest. However, serology may be performed on specimens collected up to 12 weeks following cough onset. There are several serologic tests with varying accuracy, so MDH staff can work with providers to interpret results at the time of report.
Tests not recommended for confirming pertussis
Direct Fluorescent Antibody (DFA) is a rapid laboratory diagnostic test that provides results within 1-2 days. Because of low sensitivity and variable specificity, DFA is not considered reliable for disease confirmation of B. pertussis.
Submitting specimens and isolates to the MDH Public Health Laboratory (MDH-PHL)
MDH-PHL has limited ability to perform PCR and serology for unique situations. If you would like to submit specimens to the MDH-PHL, call the vaccine-preventable disease unit at 651-201-5414 for instructions.
To request a pertussis test kit, complete the VPD Test Kit Requests form.
All positive pertussis isolates should be sent to MDH-PHL. Please contact the lab for instructions at 651-201-4953.
