2024 Public Health Laboratory Annual Report
Krabbe Disease Added to Minnesota Newborn Screening Panel
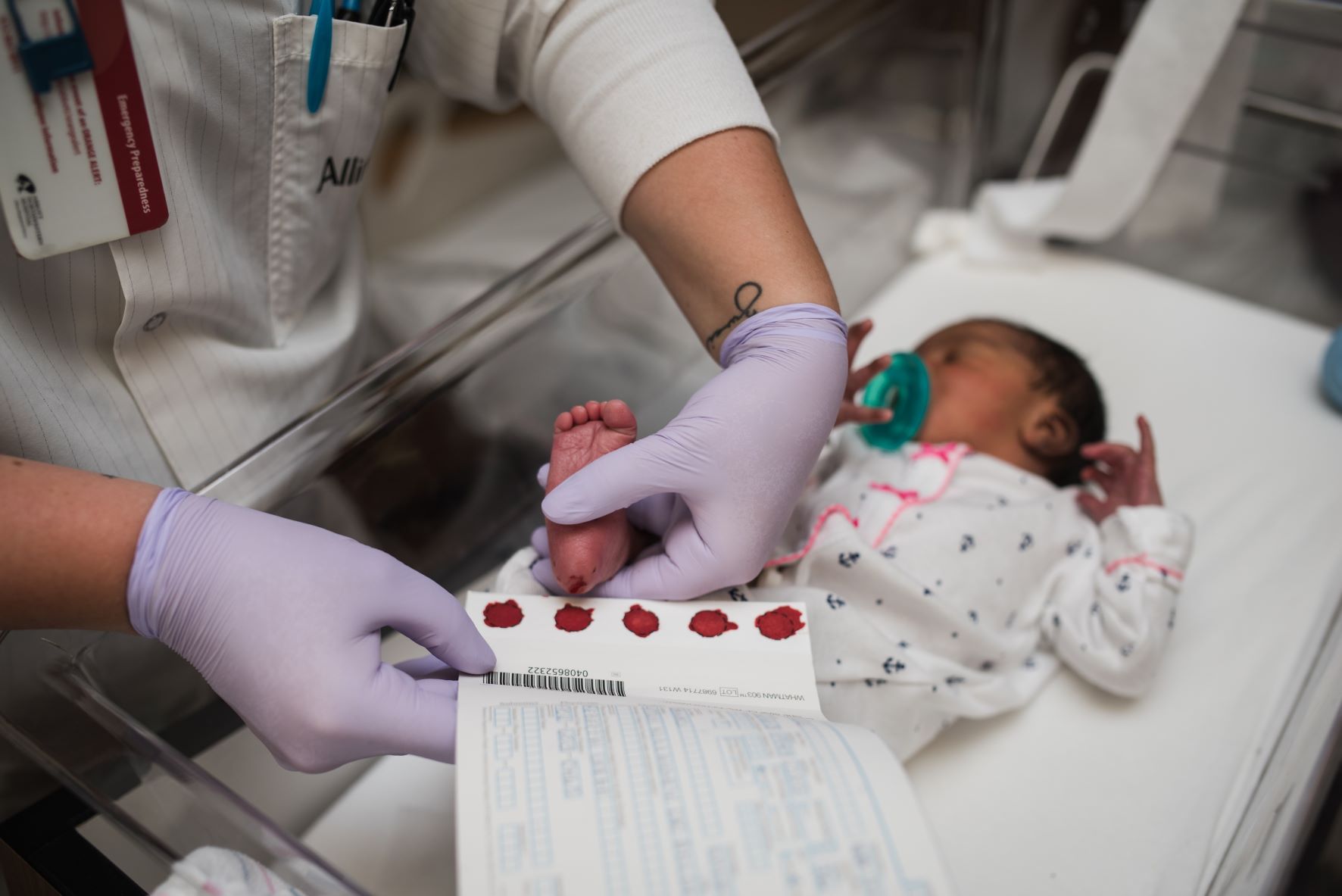 In February 2024, the Minnesota Newborn Screening Program began testing samples for Krabbe disease. As with many conditions that the program screens for, indications of Krabbe disease can be detected in a blood sample taken from the heel of a baby within 48 hours of its birth.
In February 2024, the Minnesota Newborn Screening Program began testing samples for Krabbe disease. As with many conditions that the program screens for, indications of Krabbe disease can be detected in a blood sample taken from the heel of a baby within 48 hours of its birth.
The urgency of Krabbe disease screening
Krabbe disease is a congenital, degenerative condition in which brain cells are rapidly destroyed. If left untreated, it results in paralysis, deafness, intellectual disability, and premature death.
| Infantile Krabbe Disease | |
|---|---|
| Onset | Within first two months of life |
| Symptoms | Irritability, seizures, blindness, deafness, and death within the first two years of life |
| Frequency | 1 in 250,000 live births in the United States |
| Treatment | Supportive therapies and management like physical therapy and medications. Stem cell transplant is available for infantile cases and best if given before symptoms start. |
Because Krabbe disease causes such severe and immediate health problems, a single day can make a tremendous difference in the life of an afflicted baby. No other condition on the Newborn Screening Panel necessitates more urgency. The Newborn Screening Program has systems in place to rapidly inform health care providers about the screening result so they can order further testing and plan treatments.
Health care providers unfortunately have few options for treating Krabbe disease. The current standard treatment option is hematopoietic stem cell transplantation (HSCT). In HSCT, stem cells from a healthy donor are transplanted to help the brain produce healthy cells. HSCT is much less effective if the child is already experiencing symptoms, however, and it should be done within 30 days of the diagnosis of Krabbe disease. There is a significant risk of mortality from the HSCT procedure, but it can greatly extend the length and quality of life.
Researchers are currently investigating other possible treatments for Krabbe disease, such as gene therapy, enzyme replacement therapy, and anti-inflammatory therapy. Otherwise, the primary option is palliative care, i.e., physical therapy, occupational therapy, and anti-seizure medications that alleviate symptoms.
Early results from screening
Krabbe disease can present in various forms. Infantile Krabbe disease begins affecting a child at birth, while the late-onset type can start causing symptoms at any point from 1 year old to middle-aged adulthood.
At this writing, the Minnesota Newborn Screening Program has already helped identify one case of infantile Krabbe disease, with another possible late-onset case undergoing confirmation. According to the Centers for Disease Control and Prevention, Krabbe disease occurs in 1 in 250,000 live births in the United States.
Adding Krabbe disease to the Newborn Screening Panel
 The Rosenau Family Research Foundation (formerly known as The Legacy of Angels Foundation) has spent several years lobbying for Krabbe disease to be included in the federal Recommended Uniform Screening Panel (RUSP). The RUSP is a list of conditions that the Secretary of the Department of Health and Human Services (HHS) recommends, but does not require, states to include in their newborn screening panels.
The Rosenau Family Research Foundation (formerly known as The Legacy of Angels Foundation) has spent several years lobbying for Krabbe disease to be included in the federal Recommended Uniform Screening Panel (RUSP). The RUSP is a list of conditions that the Secretary of the Department of Health and Human Services (HHS) recommends, but does not require, states to include in their newborn screening panels.
Before the federal Recommended Uniform Screening Panel (RUSP) added Krabbe disease as a core condition of the RUSP, Minnesota added the condition to its own Newborn Screening Panel. The Minnesota Newborn Screening Panel is one of the most expansive in the nation, encompassing more than 60 conditions.
The Minnesota Newborn Screening program is currently working to add testing for Duchenne muscular dystrophy (DMD), guanidinoacetate methyltransferase (GAMT) deficiency and Mucopolysaccharidosis Type II (MPS II) . Other conditions are under review for possible addition to the panel. The Minnesota Newborn Screening Program is constantly seeking to help more families discover treatable conditions in their newborns as early as possible.
Return to the main 2024 Annual Report page.
