Annual Summary of Disease Activity:
Disease Control Newsletter (DCN)
Related Topics
Contact Info
Streptococcus pneumoniae Invasive Disease, 2000
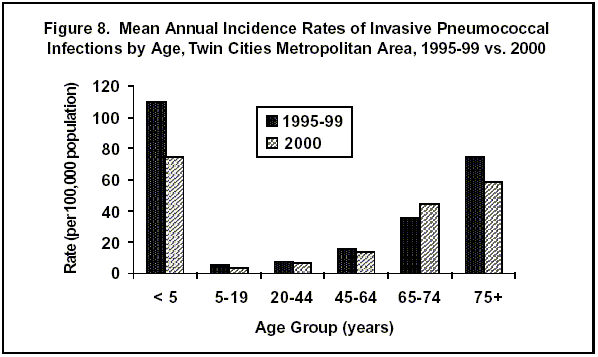
In 2000, 439 cases of invasive Streptococcus pneumoniae infection were reported among residents of the seven-county Twin Cities metropolitan area. These cases were reported as part of active, laboratory-based surveillance conducted by the EIP. Rates of invasive disease varied considerably by age; the highest rates of illness occurred in children 6 to 18 months of age, with the next highest rates among adults 75 years of age or older. In 2000, the rate of infection among children younger than 5 years dropped to 74 per 100,000 population. In this age group, the mean annual incidence from April 1995 to December 1999 was 110 per 100,000 (Figure 8). Rates among adults 75 years of age or older also declined somewhat; the rate in 2000 was 59 per 100,000, compared to a mean annual incidence of 74 per 100,000 in this age group from April 1995 to December 1999. In the first half of 2000, the overall number of cases (269) was close to the total seen on average (268) during the months of January through June from 1996 to 1999. In the first half of 2000, the number of cases in children less than 5 years of age also was close to the average from the 4 prior years. In the second half of 2000, the number of cases among children less than 5 years of age was lower than the average number of cases in each age group reported from July to December in 1996-99. There were 39 cases among children 1 year of age or younger and 14 cases among children 2 to 4 years of age.
In March 2000, a new pediatric polysaccharide-protein conjugate vaccine (Prevnar TM) covering seven pneumococcal serotypes (PCV-7) was licensed for use; the ACIP published recommendations for use of the vaccine in October 2000 (http://www.cdc.gov/nip/publications/ACIP-list.htm). The vaccine is recommended for all children under 2 years of age as well as children 2 to 4 years of age (through 59 months) with chronic medical problems (especially sickle cell disease, asplenia, or HIV infection). PCV-7 also should be considered for use in children 2 to 4 years of age who belong to one or more groups at higher risk of pneumococcal disease (i.e., children 24 to 35 months of age; children of Alaskan Native, American Indian, or African American descent, and children in daycare for 4 or more hours per week with two or more unrelated children). The decrease in incidence of invasive pneumococcal disease among children less than 5 years of age likely is attributable to use of this vaccine. Reduced rates among these young children and the elderly also may have been influenced by a relatively mild 2000-2001 influenza season.
Pneumonia accounted for 49% of the invasive pneumococcal infections in 2000, including those accompanied by bacteremia or isolation of pneumococci from another sterile site such as pleural fluid. Meningitis occurred in 21 (5%) cases, and bacteremia without another known focus of infection occurred in 169 (38%). Deaths during hospitalization or the episode of illness occurred for 31 (7%) cases which is similar to the proportion seen in prior years.
Among 136 isolates from children less than 5 years of age in 2000, 70% represented serotypes included in the PCV-7 vaccine. Among 124 isolates from adults 65 years of age or older, 85% were serotypes included in the 23-valent polysaccharide pneumococcal vaccine. This vaccine has been in use since 1983 and is recommended for use among adults 65 years of age or older and adults and children (over 2 years of age) with certain chronic medical conditions. ACIP recommendations for both pneumococcal conjugate and polysaccharide vaccines comment on use of polysaccharide vaccine.
In 2000, penicillin-resistance among invasive pneumococcal isolates submitted through EIP surveillance continued to rise. Compared with 1995 when 7% (22/305) of isolates were penicillin-resistant, 17% (68/411) of isolates in 2000 were resistant to penicillin (i.e., minimum inhibitory concentrations ≥2 ug/ml-high level resistance). Resistance to other antibiotics also has risen; the percentage of isolates resistant to two or more drug classes rose from 11% (33/305) in 1995 to 24% (98/411) in 2000. Among all antibiotics for which susceptibility data are monitored, resistance to erythromycin has risen most rapidly; 26% (105/411) of isolates in 2000 were erythromycin-resistant, compared to 8% (23/305) of isolates in 1995. Among 33 penicillin-resistant isolates from children less than 5 years of age in 2000, 29 (88%) represented serotypes included in the PCV-7 vaccine; among penicillin non-susceptible isolates from this age group, 85% (39/46) were serotypes included in the vaccine. Among 18 penicillin-resistant isolates from adults aged 65 years of age or older in 2000, 15 (83%) were serotypes included in PPV-23; among penicillin non-susceptible isolates from this age group, 78% (25/32) were serotypes included in the vaccine. Susceptibility data for invasive pneumococcal isolates collected in the seven-county metropolitan area as part of active surveillance from 1995 to 2000 are available on the MDH Pneumococcal Disease (Streptococcus pneumoniae) web site .
- For up to date information see>> Streptococcus pneumoniae (Pneumococcal Disease)
- Full issue>> Annual Summary of Communicable Diseases Reported to the Minnesota Department of Health, 2000